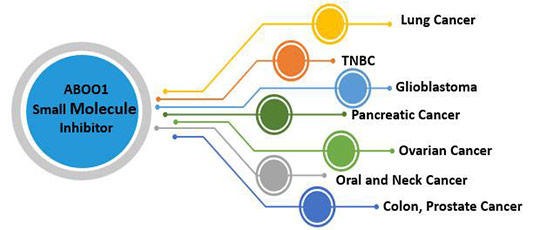
AB001 Unique Features
AB001’s important therapeutic is its binding with RNA. Due to the femtogram and picogram levels of AB001 achieved in plasma, AB001 enters the target cells and alters the function of RNA. At the transcriptional level, AB001 binds in the binding pockets of RNA and blocks its transcriptional activity which leads to prevention of tumorigenesis. At the molecular level, AB001 alters micro-RNA (miRNA) and regulates its gene function.
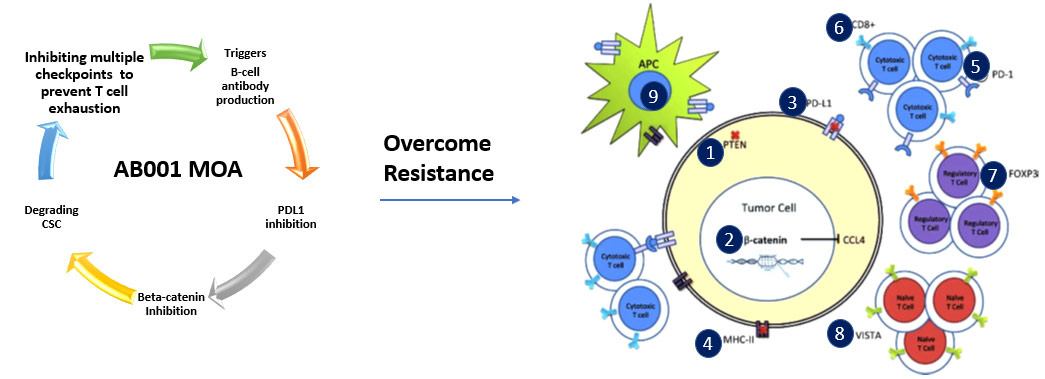
Additional mechanisms of action include the following:
- Negligible toxicity and no observable side effects
- binding to erythrocytes with greater affinity than plasma thereby improving in vivocirculation time
- Targets only cancer cells. No harm to normal cells
- Targets cancer stem cells and degrades them
- Inhibits multiple checkpoints including PD-L1
- Triggers apoptosis via MEK/JAK/STAT via the PI3K pathway
- Safely Inhibits β-catenin transcriptional activity to overcome the resistance and relapse
- No relapse has been observed in vivo
What is the difference between AB001 and other PD-1/PD-L1 inhibitors?
PD-1 inhibitors result in creating a number of serious side effects. AB001 is a B cell-centric and T cell augmentation small molecule which works on both B cells and T cells. Other check point inhibitors deal primarily with T cell empowerment. AB001 induces B cells for the production of tumor clearing antibodies, predominantly IgG1.
“B cells are at the centre of the adaptive humoral immune system … directed against invasive pathogens.” — British Society for Immunology
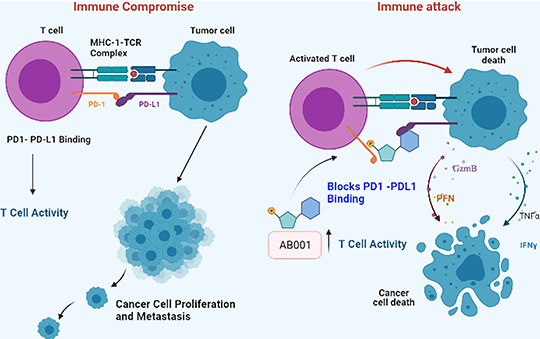
AB001 PD1/PDL1 Inhibition
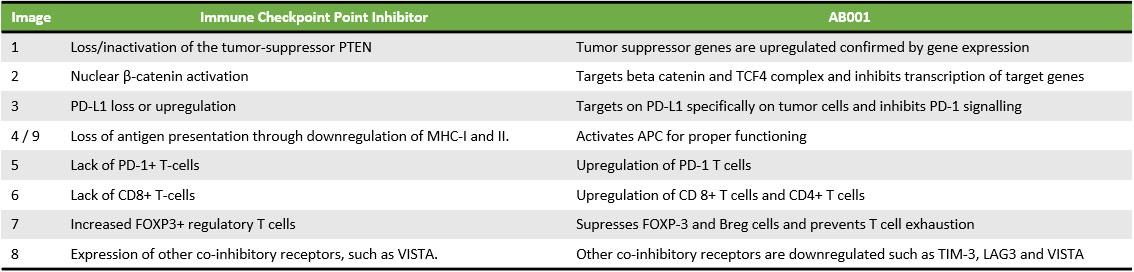
AB001 is a novel small molecule inhibitor that actively targets multiple pathways in cancer cells. It targets and binds specifically with PD-L1 on tumor cells and inhibits the downstream signaling of the PD-1 pathway that can restore the normal function of T cells.
Blockage of PD-1/PD-L1 interaction results in the reversal of the exhausted T-cell phenotype and normalization of antitumor response, providing the rationale of targeted therapy. It also stimulates B cells to secrete tumor-eliminating antibodies and inhibits the suppression of regulatory cells by Inhibiting IL-10 and TGF beta.
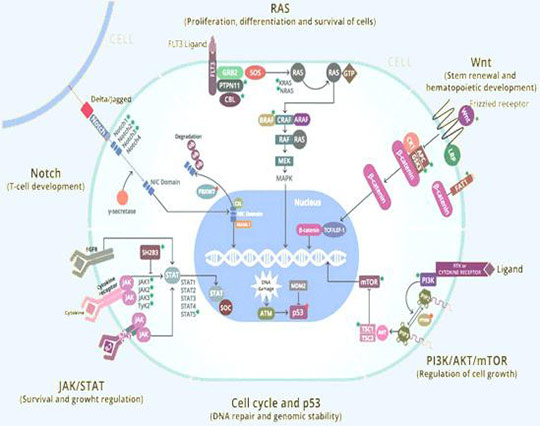
Pathways Inhibited by AB001
Being a small molecule it easily transverses through the plasma membrane and targets multicellular pathways such as WNT/β Catenin, PI3K, MAPK pathway, which are mainly involved in cellular proliferation survival and resistance.
None of the currently available chemo drugs target cancer stem cells due to their slow-growing ability, fast differentiation and expansion, which are responsible for relapse and recurrence.
AB001 is the first small molecule that eliminates cancer stem cells from the root via the beta-catenin pathway and degrades them.
Furthermore, toxicity studies prove the safety of the molecule making it a promising alternative candidate for chemotherapy.
AB001 Overcoming Resistance of Immune Checkpoint Inhibitors
AB001 B Cell-centric & T Cell Augmentation: Novel Immuno-Oncology
- AB001 induces B cells for the production of tumor clearing antibodies, predominantly IgG1:
- mediating antibody dependent cell-mediated cytotoxicity (ADCC)
- mediating antibody dependent cell-mediated phagocytosis (ADCP)
- facilitating complement activation
- Activation of CD4 helper cells and CD8 cytotoxic T cells
- Inhibition of CD4 regulatory T cells and B regulatory cells
- Inhibition of IL-10, TGF beta which inhibit effector function of T cells
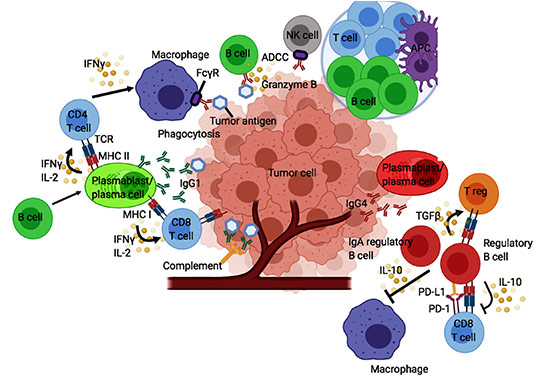
“B cells can also promote differentiation of Th1, Cytotoxic T-cell and can aid in better T cell mediated immune response. The release of Granzyme B can directly kill cancer cells and support the tumor suppressive actions of B-cells in tumor microenvironment. Release of IFNα can stimulate TLR agonist to kill tumor cells through the TRAIL signaling activity” – Sarvaria, Cellular & Molecular Immunology, Aug 2017